41 cautionary and advisory labels for medicines
bnf.nice.org.uk › drugs › metformin-hydrochlorideMedicinal forms | Metformin hydrochloride | Drugs | BNF | NICE Cautionary and advisory labels. Label 21 . Take with or just after food, or a meal. Cymerwch gyda neu ar ôl bwyd. Label 25 . Swallow this medicine whole. Do not chew or crush. Llyncwch yn gyfan. Peidiwch â chnoi neu falu’n fân. Show Diagemet XL 500mg tablets Genus Pharmaceuticals Ltd Show Cautionary and advisory labels. Label 21 About | BNFC | NICE Nurse Prescribers' Advisory Group; How BNF Publications are constructed; How to use BNF Publications online; Frequently asked questions for the BNF and BNF for Children—general; Frequently asked questions—clinical; Approximate Conversions and Units; Abbreviations and Symbols; Medicines Information Services; Labels; Guidance for cautionary ...
Cautionary and advisory labels for medicines - Everything2.com These are the code numbers and their meanings for the cautionary labels used by pharmacist s when dispensing medicine s in the UK. Extra counselling may be given with relation to age, experience , background and understanding of the patient. Warning. May cause drowsiness

Cautionary and advisory labels for medicines
en.wikipedia.org › wiki › Boxed_warningBoxed warning - Wikipedia This advisory was associated with a decrease in use of antipsychotics, especially in elderly patients with dementia. As of 2006, natalizumab (marketed as Tysabri) received a boxed warning on its packaging due to increased risk of developing progressive multifocal leukoencephalopathy (PML). Tysabri was pulled from the market in 2004, shortly ... Cautionary and advisory labels | About | BNF | NICE Medicines Information Services; Labels; Guidance for cautionary and advisory labels; On this page. Label 1; Label 2; Label 3; Label 4; Label 5; View guidance for cautionary and advisory labels. Label 1. Warning: This medicine may make you sleepy. Rhybudd: Gall y feddyginiaeth hon eich gwneud yn gysglyd. To be used on preparations for children containing … Label Statements Database - Medsafe This database lists the warning and advisory statements that are required on medicine and related product labels under regulations 13 (1) (i) and 14 (1) (f) of the Medicines Regulations 1984. Words of a similar meaning to the statements in the database may be used and individual statements may be combined provided the intent is not changed.
Cautionary and advisory labels for medicines. Cautionary advisory labels - Australian Pharmacist The dispensing pharmacist has dispensed the patient's discharge medications and has labelled simvastatin with cautionary advisory label 21 (Special handling and disposal required - ask your pharmacist) and label A (Swallow whole do not crush or chew). You are concerned that these labels may alarm and/or confuse the patient. What should you do? 50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings. The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even ... Cautionary And Advisory Labels - Medico Pak Cautionary And Advisory Labels. These labels are available in eye-catching fluro orange with different statements or instructions. We recommend using these labels to assist care facilities to maximise the safety by affixing appropriate labels to the pack where necessary. ... As Required Medication: 200: 38071: Look For A Second Pack: 200: 38072 ... Boxed warning - Wikipedia In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. The FDA can require a pharmaceutical company to place a boxed warning on the …
PDF Revisions to APF24 Cautionary advisory labels Revisions to Table A2. Recommended cautionary advisory labels for medicines Revised CAL recommendations for the following medicines are shown in the table below. Medicine Revised ancillary label (number) and/or additional instruction (letter) Abacavir Oral solution: 7b (60 days), 12†, 21 Tablet: 12†, 21, A Aciclovir Eye ointment: 7b (28 ... Medicinal forms | Tezacaftor with ivacaftor and elexacaftor | Drugs ... Cautionary and advisory labels. Label 25 . Swallow this medicine whole. Do not chew or crush. Llyncwch yn gyfan. Peidiwch â chnoi neu falu'n fân. Show (black triangle) Kaftrio 37.5mg/25mg/50mg tablets Vertex Pharmaceuticals (Europe) Ltd Show Cautionary and advisory labels. Label 25 . Swallow this medicine whole. Do not chew or crush ... StirlingFildes | HealthCare Cautionary Advisory Labels The StirlingFildes Cautionary Advisory Label (CALs) system produced by the PSA is a valuable labelling system designed for helping pharmacists to fulfil their legal and professional obligations, promote quality use of medicines, and provide optimal outcomes for consumers. bnf.nice.org.uk › about › labelsCautionary and advisory labels | About | BNF | NICE To be used on preparations containing ofloxacin and some other quinolones, doxycycline, lymecycline, minocycline, and penicillamine. These drugs chelate calcium, iron, and zinc and are less well absorbed when taken with calcium-containing antacids or preparations containing iron or zinc.
Medicinal forms | Mefloquine | Drugs | BNFC | NICE Cautionary and advisory labels. Label 10 . Warning: Read the additional information given with this medicine. Rhybudd: Darllenwch y wybodaeth ychwanegol gyda'r feddyginiaeth hon. Label 21 . Take with or just after food, or a meal. Cymerwch gyda neu ar ôl bwyd. Label 27 . Take with a full glass of water. Cymerwch gyda llond gwydr o ddŵr. Show PDF National standard for labelling - Safety and Quality 3.6 Cautionary advisory labels 16 4 Standards for dispensed medicine labels 17 Standard 1: Prominently display the information that consumers need to take their medicine ... The dispensed medicine label provides customised information about the medicine and how the consumer should use it, at the point of use. The label is especially important ... Cautionary and advisory labels for medicines - [PPTX Powerpoint] Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counseling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know. Kiran Sharma, KIET School of Pharmacy 11/12/2013 2 Consumer Updates | FDA The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
bnfc.nice.org.uk › aboutAbout | BNFC | NICE Nurse Prescribers' Advisory Group; How BNF Publications are constructed; How to use BNF Publications online; Frequently asked questions for the BNF and BNF for Children—general; Frequently asked questions—clinical; Approximate Conversions and Units; Abbreviations and Symbols; Medicines Information Services; Labels; Guidance for cautionary ...
Labels on medicines and poisons - Department of Health Labels on medicines and poisons All medicines and poisons containers must be labelled so as to clearly identify the contents. This is important to prevent inadvertent consumption and poisoning. Labels must meet uniform Australian standards on text size, language and warnings. Labels on poisons Dispensing labels Labels on medicines More information
StirlingFildes | Printing, Packaging and Consumables Cautionary & Advisory Labels. Buy a mixture of 12 or more labels and save. The savings will be calculated on your invoice. Product Image Product Description & Code Product Details; Warning Label No. 1 Code: WARN 1: 1,000 per box 46 x 16mm: Warning Label No. 1A Code: WARN 1A:
PDF cautionary advisory labels - Openbook Howden cautionary advisory labels OBH 18642 CAL's are a valuable tool for helping pharmacists to fulfil their legal and professional obligations, promote quality use of medicines, and provide optimal outcomes for consumers. Reproduced with the permission of the Pharmaceutical Society of Australia. Sold in dispenser boxes of 1000.
Safer dispensing labels for prescription medicines Dispensing labels on prescribed medicines provide administration instructions and important warnings. These remain with the consumer after the initial consultation when some of the confusion and worry frequently associated with illness has started to recede. ... Australia has a national 'standard icon system' for cautionary advisory labels ...
› consumers › consumer-updatesConsumer Updates | FDA Consumer Updates Science-based health and safety information you can trust.

Alcohol, drugs and medicines - Safe driving - Safety and rules - Roads - Roads and Maritime Services
Cautionary and advisory label - sensagent Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory labels.
FDA Drug Safety Communication FDA Briefing Information for the February 10-11, 2014 Joint Meeting of the Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee. Available from:. Accessed December ...
Cautionary_and_advisory_label - chemeurope.com Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counselling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know. Label Wording and Warnings
Cautionary and advisory labels for medicines - SlideShare Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counseling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know. Kiran Sharma, KIET School of Pharmacy 11/12/2013 2 3.
Medicinal forms | Selumetinib | Drugs | BNFC | NICE Cautionary and advisory labels. Label 25 . Swallow this medicine whole. Do not chew or crush. Llyncwch yn gyfan. Peidiwch â chnoi neu falu'n fân. Excipients. May contain vitamin e. Show (black triangle) Koselugo 10mg capsules AstraZeneca UK Ltd Show Cautionary and advisory labels. Label 25 . Swallow this medicine whole. Do not chew or crush ...
PDF Revisions to APF25 Cautionary advisory labels Cautionary advisory labels Revisions to Table 2.2 Recommended cautionary advisory labels for medicines Revised CAL recommendations for the following medicines are shown in the table below. Medicine Revised ancillary label (number) and/or additional instruction (letter) Acetazolamide 8, 10a, 12 Adalimumab 6 (except syringe in use), 7b*, 13
Required Advisory Statements for Medicine Labels (RASML) RASML No. 6. RASML No. 6 was published on 1 January 2022 as Schedule 1 to the 2021 Specification and comes into full effect on 1 July 2023 after the 18-month transition period. During the transition period, labels may comply with either RASML No. 5 or RASML No. 6. RASML No. 6 includes the new and amended statements that were the subject of ...
Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication. Auxiliary labels provide information which can ...
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication FDA Briefing Information for the February 10-11, 2014 Joint Meeting of the Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee. Available from:. Accessed December ...
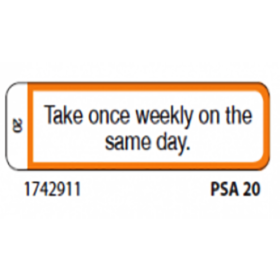



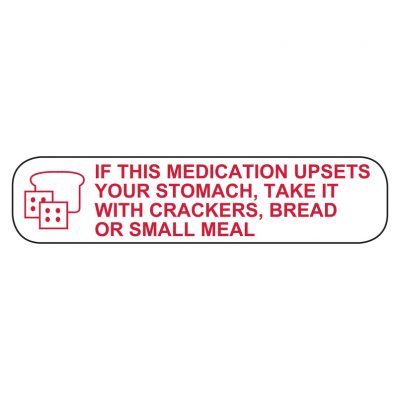





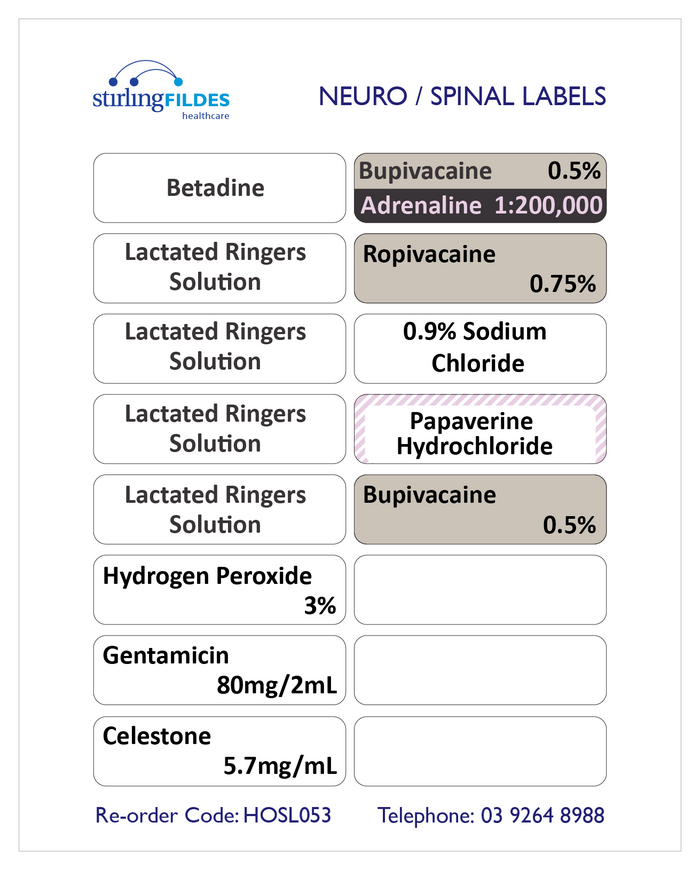

Post a Comment for "41 cautionary and advisory labels for medicines"