44 health claims on food labels are standardized and regulated
EOF Health Claims on Food Labels - Consumer Reports Specifically, grass-fed meat and dairy has a more healthful ratio of omega-6 polyunsaturated fatty acids to omega-3s. Too much omega-6 fat in your diet can cause inflammation, which may be a ...
Food labelling and packaging: Nutrition, health claims and supplement ... Nutrition, health claims and supplement labelling, Nutrition labelling, You must follow nutrition labelling information rules for all pre-packed products unless both of the following apply: you're...
Health claims on food labels are standardized and regulated
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and › inspection › import-exportPHIS Components | Food Safety and Inspection Service Jun 13, 2022 · FSIS is providing foreign governments, as well as importers and brokers of meat, poultry, and egg products, the following letters regarding the final rule, Electronic Import Inspection Application and Certification of Imported Products and Foreign Establishments; Amendments To Facilitate the Public Health Information System (PHIS) and Other Changes to Import Inspection Regulations. en.wikipedia.org › wiki › Organic_foodOrganic food - Wikipedia In the United States, organic production is managed in accordance with the Organic Foods Production Act of 1990 (OFPA) and regulations in Title 7, Part 205 of the Code of Federal Regulations to respond to site-specific conditions by integrating cultural, biological, and mechanical practices that foster cycling of resources, promote ecological balance, and conserve biodiversity.
Health claims on food labels are standardized and regulated. Questions and Answers on Health Claims in Food Labeling The Nutrition Labeling and Education Act of 1990 (NLEA) directed FDA to issue regulations providing for the use of health claims. All health claims must undergo review by the FDA through a petition... Factual Food Labels: Health Claims - University of Texas at Austin Health Claims. In 1990, the Nutrition Labeling and Education Act allowed claims that related a specific food component (e.g., oats) to lowered disease risk (e.g., reduced cholesterol) to be printed on the label of a food product. For example, if a company wants to place a health claim on their food packaging, such as "Heart Healthy," they must ... Nutrition and health claims: guidance to compliance with Regulation (EC ... 1.6 Nutrition and health claims for food being placed on the market within the EU from 1 January 2021, Regulation (EC) No. 1924/2006 sets out the legal framework for businesses wanting to make... Regulating health claims on food labels using nutrient ... - PubMed Objective: Proposed Australian regulation of claims on food labels includes requirements for products carrying a health claim to meet nutrient profiling criteria. This would not apply to nutrition content claims. The present study investigated the number and healthiness of products carrying claims and the impact of the proposed regulation.
Nutrition, health and related claims - Food Standards Health claims, You can only base health claims on food-health relationships that have been substantiated according to Standard 1.2.7. All health claims must be supported by scientific evidence to the same degree of certainty, whether they are pre-approved by us or self-substantiated by food businesses. General level health claims, Food Label Claims: What You Can and Can't Trust - WebMD Electronic Code of Federal Regulations: "Country of Origin Labeling for Fish and Shellfish," "Food Labeling: Subpart E — Specific Requirements for Health Claims." FDA: "Label Claims for ... Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ... abcnews.go.com › healthHealth News | Latest Medical, Nutrition, Fitness News - ABC ... Aug 24, 2022 · Get the latest health news, diet & fitness information, medical research, health care trends and health issues that affect you and your family on ABCNews.com
› food › information-consumers-usingQuestions and Answers on Dietary Supplements | FDA May 06, 2022 · Among the claims that can be used on dietary supplement labels are three categories of claims that are defined by the FD&C Act and FDA regulations: health claims (claims about the relationship ... canadagazette.gc.ca › rp-pr › p2/2022/2022-07-06Regulations Amending the Natural Health Products Regulations ... Jul 06, 2022 · In these jurisdictions, facts tables containing important information are already required for similar products that make health claims. For example, in the U.S., a product regulated as a food requires a Nutrition Facts table, a dietary supplement requires a Supplement Facts table, and a drug requires a Drug Facts table. Nutrition and Health Claims - Food Safety Union rules on nutrition and health claims have been established by Regulation (EC) No 1924/2006.The Regulation started to apply on 1 July 2007. This regulation is the legal framework used by food business operators when they want to highlight the particular beneficial effects of their products, in relation to health and nutrition, on the product label or in its advertising. Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard, Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Health Claims - Canada.ca The Food Directorate of Health Canada is responsible for the development of policies, regulations and standards that relate to the use of health claims on foods. Health claims on foods may help people make informed decisions about food choices provided they are truthful and not misleading.
5 Understanding Food Labels and Health Claims - Maricopa No labels can make claims of diagnosis, cures, treatment, or disease prevention. If you find food or drinks that make wild claims of curing or treating a disease or symptom (or making you lose weight or gain muscle), note that it is NOT TRUE. These are not valid or allowed claims on food labels. Test You Knowledge, Allergy Warnings,
Nutrient Claims on Food Labels - Clemson University Lean Claims. Lean. Contains less than 10 grams total fat, 4.5 grams or less saturated fat, and less than 95 milligrams cholesterol. Extra lean. Contains less than 5 grams total fat, less than 2 grams saturated fat, and less than 95 milligrams cholesterol. *compared to the reference, or regular, food this would replace.
› health › using-dietaryUsing Dietary Supplements Wisely - NCCIH However, some types of claims related to health or the way that the product affects the structure or function of the body may appear on dietary supplement labels. Health claims describe a relationship between a substance in the supplement and reduced risk of a disease or condition. They must be based on scientific evidence.
› food › nutrition-education-resourcesGluten and Food Labeling | FDA - U.S. Food and Drug ... The gluten-free labeling regulation gives consumers a standardized tool for managing their health and dietary intake — especially those with celiac disease, an auto-immune reaction to eating ...
CH 2 - NUTRITION Flashcards | Quizlet Structure/function claims on dietary supplement labels are not regulated as rigorously as health claims. TRUE. Serving sizes are standardized on food labels to allow consumers to compare products. ... Nutrient content and health claims are approved by the: FDA. A _____ claim refers to a relationship between a nutrient, food, or dietary ...
Health claims on food labels - PubMed Health claims on food labels, Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there was no requirement that nutrition information be provided. The Nutrition Labeling and Education Act of 1990 (NLEA), passed on November 8, 1990, mandated the Food and Drug Administrati …,
en.wikipedia.org › wiki › Organic_foodOrganic food - Wikipedia In the United States, organic production is managed in accordance with the Organic Foods Production Act of 1990 (OFPA) and regulations in Title 7, Part 205 of the Code of Federal Regulations to respond to site-specific conditions by integrating cultural, biological, and mechanical practices that foster cycling of resources, promote ecological balance, and conserve biodiversity.
› inspection › import-exportPHIS Components | Food Safety and Inspection Service Jun 13, 2022 · FSIS is providing foreign governments, as well as importers and brokers of meat, poultry, and egg products, the following letters regarding the final rule, Electronic Import Inspection Application and Certification of Imported Products and Foreign Establishments; Amendments To Facilitate the Public Health Information System (PHIS) and Other Changes to Import Inspection Regulations.
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and




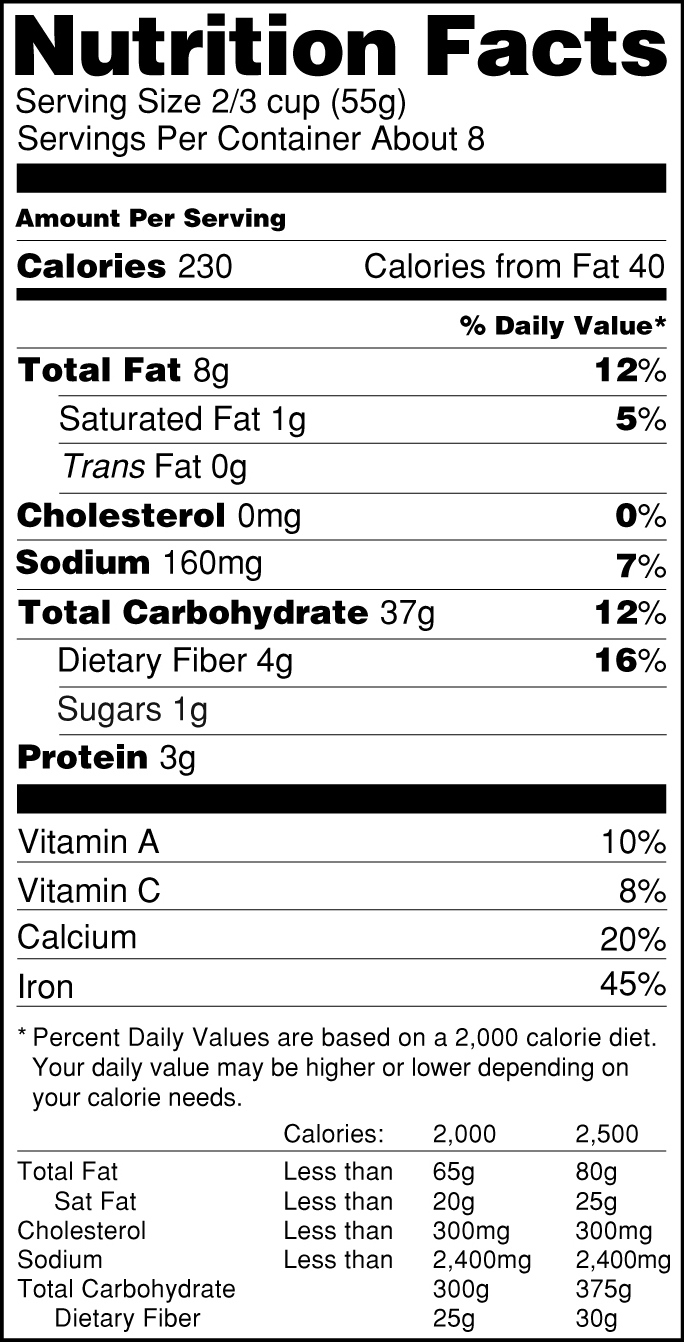






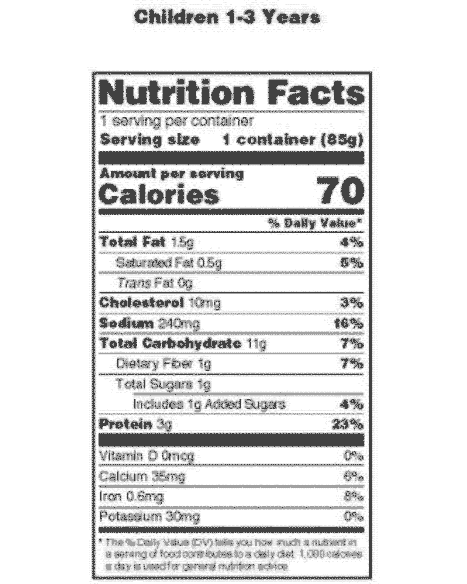

:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)






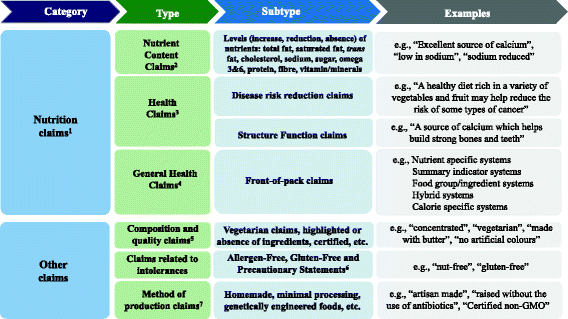






:max_bytes(150000):strip_icc()/oreo-sugar-free-400x400-1679202a775547cabf7be9d8385198a6.jpg)

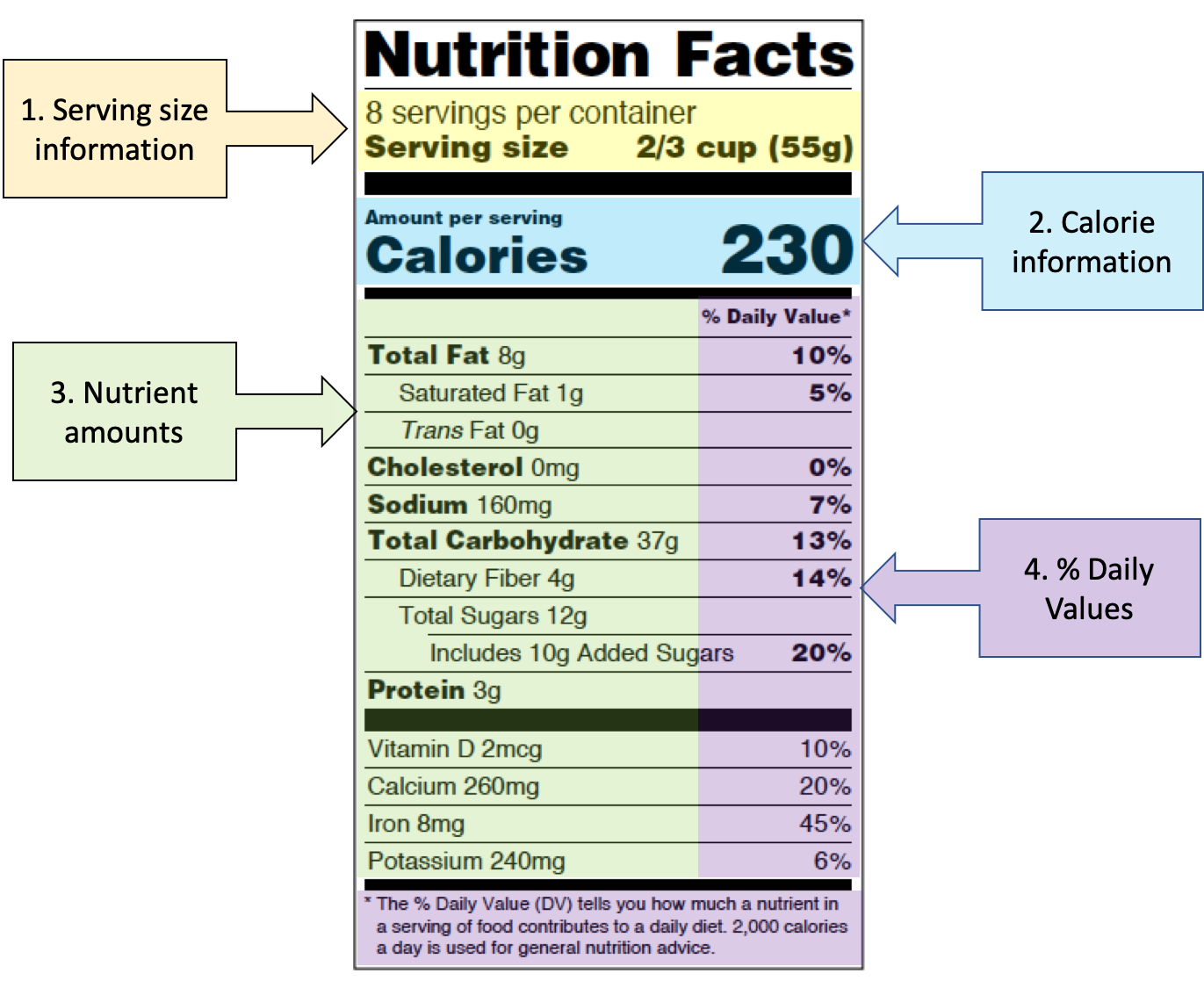









Post a Comment for "44 health claims on food labels are standardized and regulated"