42 fda structured product labels
SPM & SPL Labeling, US FDA, Health Canada - AXSource Structured Product Labeling is a labelling format based on XML and Health Level Seven (HL7) SPL standards and controlled vocabularies. Regulatory agencies such as Health Canada and FDA, define SPL rules and Regulations for labelling compliance. Nsde | Fda CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for...
Structured Product Labeling Validation Rules Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ...
Fda structured product labels
Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug. FDA SPL - Structured Product & Drug Labeling Composition Process | Reed ... Structured product labeling for both prescription and over-the-counter (OTC) drugs must incorporate an overview of the scientific information needed for the correct and effective use of the drug. The labeling is broken up into sections including explanations for use (prescription drugs) or purpose (OTC drugs), adverse effects, and more. Indexing Structured Product Labeling | FDA Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) will index the...
Fda structured product labels. Comirnaty Not Available - by Warner Mendenhall - CovidLawCast.Com NDCs are listed per FDA Structured Product Label (SPL) document for the BLA licensed product. ... Pfizer has provided the following statement regarding the COMINARTY branded NDCs and labels: "Pfizer received FDA BLA license on 8/23/2021 for its COVID-19 vaccine for use in individuals 16 and older (COMIRNATY). At that time, the FDA published a ... Food Labeling: Revision of the Nutrition and Supplement Facts Labels May 27, 2016 · Instead, we focused on how the label formats affected consumers': (1) Perceptions of the healthfulness and levels of nutrients of a product; (2) identification of which product in a pair of products was considered healthier; (3) accuracy of identifying the amount of nutrients per serving and per container and number of servings per container ... DailyMed Sep 15, 2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical … Structured Product Labeling (SPL) | Data Conversion Laboratory Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard.
Reed Tech | Best-In-Class Information-Based Solutions and Services Build and submit UDI records electronically in Structured Product Labeling (SPL) format or seek subject-matter expertise. Manage Medical Device Product Data for UDI & Syndication Serving the medical device manufacturing industry in the areas of compliance, data management and regulatory requirements experience. SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ... Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... Methods. All 2217 labels available on DailyMed were downloaded as of February 23, 2007 and fed into an open-source HL7 v3 based data-infrastructure (HL7 JavaSIG). 12 The HL7 Reference Information Model (RIM) 13 is used directly as the object model. The HL7 RIM represents medicines, packages and ingredient-substances as (physical) Entities related to each other through Roles which specify the ... Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL...
Structured Product Labeling Improves Detection of Drug-Intolerance Issues Introduction and Objective. The HL7 Structured Product Labeling (SPL) standard 1 implemented by the FDA uses the HL7 Reference Information Model (RIM) 2 to represent the chemical and physical nature of medical products and their safe and effective use. While not all of this content is available today, we enrich the 3704 available SPLs with knowledge from the SPL terminology sources, including ... 2009AA FDA Structured Product Labels Source Information SPL is a document markup standard approved by Health Level Seven (HL7) and adopted by the FDA as a mechanism for exchanging medication information. Metathesaurus Scope MTHSPL includes drug product and active substance terminology used in Structured Product Labels. MTHSPL contains approximately 9,942 drug products and 2,112 substances. UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Synopsis SPL is a document markup standard approved by Health Level Seven (HL7) and adopted by the FDA as a mechanism for exchanging medication information. Metathesaurus Scope MTHSPL includes drug product and active substance terminology used in Structured Product Labels. MTHSPL contains approximately 158,821 drug products and 21,070 substances. A dataset of 200 structured product labels annotated for adverse drug ... The Structured Product Labels (SPLs), the documents FDA uses to exchange information about drugs and other products, were manually annotated for adverse reactions at the mention level to ...
FDA-Approved COVID Shot Exists On Paper Only | NaturalHealth365 Jun 24, 2022 · (NaturalHealth365) FDA, CDC admits that no one in this country is or ever will get the FDA-approved COVID shot. ... (Pre-Authorization) or have been authorized or approved by the FDA under EUA or BLA License and may be included in FDA NDC files and Structured Product Labels (SPL). ... It is so curious why there was such a rush to approve this ...
Package Type | FDA In this section: Structured Product Labeling Resources ... Electronic Animal Drug Product Listing Directory; Equivalence Codes; Flavor; Geopolitical Entities, Names, and Codes (GENC) ...
Managing The Device Master Record (DMR) | Arena A modern product record often includes a complex set of hundreds to thousands of structured items. Poring over thousands of rows and columns in a spreadsheet to modify data leads to errors. Rapid changes during the design and prototype phases result in a higher probability of making mistakes.
FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents....
Drug Labeling Overview - Food and Drug Administration Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns data from this...
Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The openFDA...
MTHSPL (FDA Structured Product Labeling) Source Information SPL is a document markup standard approved by Health Level Seven (HL7) and adopted by the FDA as a mechanism for exchanging medication information. RxNorm Scope MTHSPL includes drug product and substance terminology used in Structured Product Labels. Update FrequencyMTHSPL is updated daily.
NSDE | FDA Mar 31, 2022 · In this section: Structured Product Labeling Resources ... this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov. The information ...
UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Metadata Metathesaurus FDA Structured Product Labels, 2022_02_25: Short Name: FDA Structured Product Labels: Family: MTHSPL: Metathesaurus Insertion Version: 2022AA: Restriction Level: 0: Language: ENG: Context Type: License Contact: RxNorm Customer Service U.S. National Library of Medicine 8600 Rockville Pike Bethesda MD United States
Structured Product Labeling Resources | FDA Apr 06, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ...
FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently...
DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical …
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not …

![Food Labeling 101 - FDA Regulations Guide [2021] | Artwork Flow](https://uploads-ssl.webflow.com/5f59aa263c234bb74025de57/5fa4fb135e79e89d4cb98cf5_Inner-Images-7.jpg)

![FDA - Labeling - [PDF Document]](https://reader019.documents.pub/reader019/reader/2020031315/58a1ddb31a28abb6678b698b/r-1.jpg)


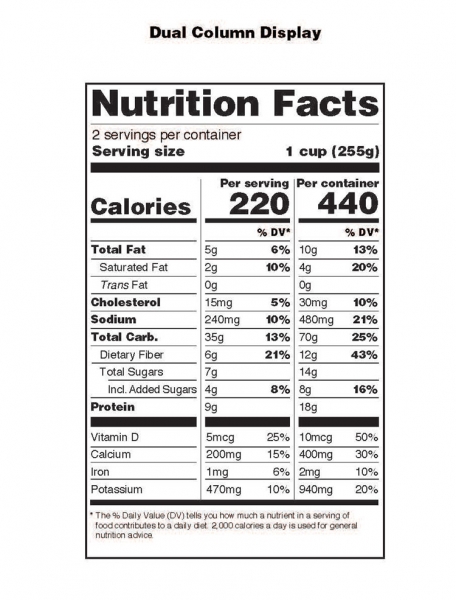
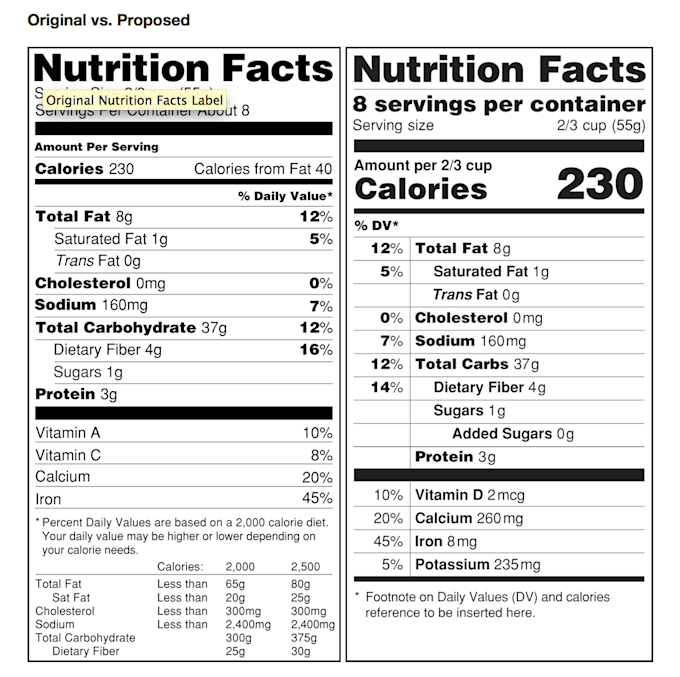




Post a Comment for "42 fda structured product labels"