43 fda approved drug labels
FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents.... Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 04/28/2022: ORIG-1: Approval Label (PDF)
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions...
Fda approved drug labels
Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Drug labels containing PGx information were obtained from Drugs@FDA and guidelines from PharmGKB were used to compare the actionability of PGx information in drug labels across therapeutic areas. The annual proportion of new drug approvals with PGx labeling has increased by nearly threefold from 10.3% (n = 3) in 2000 to 28.2% (n = 11) in 2020 ... DailyMed The DailyMed database contains 142965 labeling submitted to the Food and Drug Administration (FDA) by companies. DailyMed does not contain a complete listing of labeling for FDA-regulated products (e.g., labeling that is not submitted to the FDA). See ABOUT DAILYMED for more information. Share News DailyMed Announcements Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 12/17/2021: ORIG-1: Approval Label (PDF)
Fda approved drug labels. Search FDA Drug Labels With WIZMED | Orange Book Search Data Upon FDA drug approval, details on FDA labels are sent to multiple agencies who publish different information. Not to mention, all the competitive intelligence drug information you are seeking is redacted and located in different databases. One Search is on a mission to bring all that data to your fingertips in just one search. Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/23/2022: ORIG-1: Approval Label (PDF) FDA revises labels of SGLT2 inhibitors for diabetes to ... Mar 15, 2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ... Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for use...
Consumer Updates | FDA Jun 02, 2022 · Is It Really 'FDA Approved'? The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including ... New Drugs - List of Latest FDA Approvals 2022 - Drugs.com Company: Dermavant Sciences. Date of Approval: May 23, 2022. Treatment for: Plaque Psoriasis. Vtama (tapinarof) is a topical aryl hydrocarbon receptor (AhR) modulating agent indicated for the treatment of plaque psoriasis in adults. FDA Approves Vtama (tapinarof) Cream for the Treatment of Plaque Psoriasis in Adults - May 24, 2022. LabelCalc | Plans & Pricing | FDA Nutrition Labels Our database contains over 200,000 USDA-compiled lab-tested ingredients that combine to create your recipes. Because this method is so accurate, the FDA has approved database nutritional analysis for determining your nutrition facts. We only recommend using a lab if your ingredients change in chemical structure during processing (i.e. fermentation during brewing, or deamination in cheese making). Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web...
Seres Therapeutics Touts New Safety Data, Plans First FDA Approval For ... Seres Therapeutics Inc (NASDAQ: MCRB ) announced confirmatory results from ECOSPOR IV, an open-label study for SER-109 to prevent recurrent C. difficile infection (rCDI). The overall safety ... PDF HIGHLIGHTS OF PRESCRIBING INFORMATION - accessdata.fda.gov mutation as detected by an FDA-approved test. (1.1, 2.1) TAFINLAR is indicated, in combination with trametinib, for: • the treatment of patients with unresectable or metastatic melanoma with BRAF V600E or V600K mutations as detected by an FDA-approved test. (1.2, 2.1) • the adjuvant treatment of patients with melanoma with BRAF V600E or Overview of Cosmetic Labeling Requirements in US The following information is a brief introduction to labeling requirements. Proper labeling is an important aspect of putting a cosmetic product on the market. FDA regulates cosmetic labeling under the authority of both the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act (FPLA). Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 12/21/2020: SUPPL-34: Efficacy-Labeling Change With Clinical Data
FDA Label Search The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA,...
Prescription Drug Labeling Resources | FDA FDA's Prescription Drug Labeling Resources website provides over 150 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for...
Drugs@FDA: FDA-Approved Drugs For prescription brand-name drugs, Drugs@FDA typically includes the most recent labeling approved by the FDA (for example, Prescribing Information and FDA-approved patient labeling when available),...
Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) As of November 10, 2020, PharmGKB listed a total of five drug approvals with "recommended testing", of which two (i.e., azathioprine and thioguanine) were first approved prior to 2000 and to which biomarker information was added as part of subsequent labeling updates [ 62 ].
FDA Issues Drug Safety Communication Related to Current XELJANZ® Label ... New York, September 1, 2021 — The U.S. Food and Drug Administration (FDA) has issued a Drug Safety Communication (DSC) related to XELJANZ ® /XELJANZ XR ® (tofacitinib) and two other arthritis medicines in the same drug class, based on its completed review of the ORAL Surveillance trial. The communication is an update to the agency's DSC ...
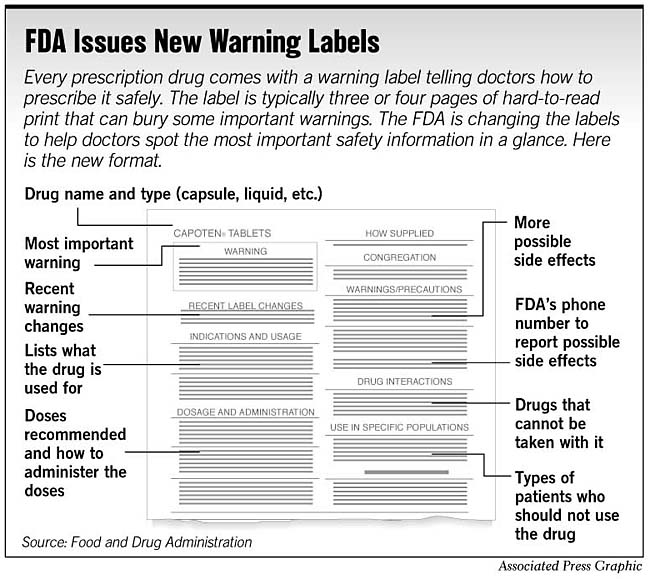
FDA Drug Labeling Product Requirements, Guidance - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. An example of FDA-approved labeling is the Professional Package Insert (PPI).
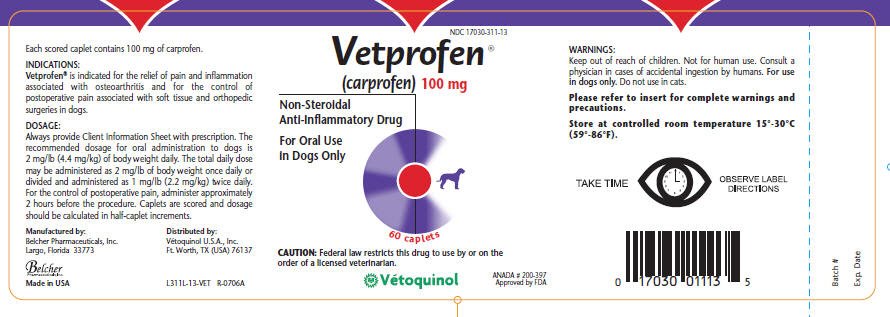
Drug Labels | FDA Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and...
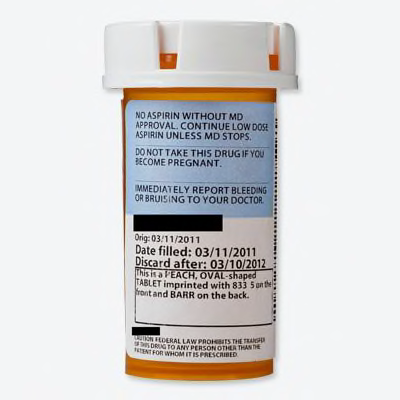
Drugs@FDA: What's in a Drug Product Label? | FDA adverse events (side effect) drug abuse and dependence. dosage and administration. use in pregnancy, use in nursing mothers. use in children and older patients. how the drug is supplied. safety ...
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 02/11/2021: ORIG-1: Approval Label (PDF)
OTC Drug Facts Label | FDA In the Federal Register of March 1999, the Food and Drug Administration published the OTC Drug Facts Label regulation. This regulation required most OTC drug products to comply with the new format...
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 08/13/2021: SUPPL-93: Labeling-Package Insert
FDA-approved drug labeling for the study of drug-induced liver injury However, assessing the DILI potential of a drug is a challenge with no existing consensus methods. We proposed a systematic classification scheme using FDA-approved drug labeling to assess the DILI potential of drugs, which yielded a benchmark dataset with 287 drugs representing a wide range of therapeutic categories and daily dosage amounts.
FDA approves Roche's Evrysdi for use in babies under two months with ... RHHBY. Basel, 31 May 2022 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that the U.S. Food and Drug Administration (FDA) has approved a label extension for Evrysdi® (risdiplam) to include babies under two months old with spinal muscular atrophy (SMA). The approval is based on interim efficacy and safety data from the RAINBOWFISH study ...
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 12/17/2021: ORIG-1: Approval Label (PDF)
DailyMed The DailyMed database contains 142965 labeling submitted to the Food and Drug Administration (FDA) by companies. DailyMed does not contain a complete listing of labeling for FDA-regulated products (e.g., labeling that is not submitted to the FDA). See ABOUT DAILYMED for more information. Share News DailyMed Announcements
Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Drug labels containing PGx information were obtained from Drugs@FDA and guidelines from PharmGKB were used to compare the actionability of PGx information in drug labels across therapeutic areas. The annual proportion of new drug approvals with PGx labeling has increased by nearly threefold from 10.3% (n = 3) in 2000 to 28.2% (n = 11) in 2020 ...








Post a Comment for "43 fda approved drug labels"